Skills:
Prototyping
Mechanical Design (CAD)
Protocol Design
Training and supervising
510(k) FDA Submission
Collaborators:
Moxxly
Flow; Service Life Testing Equipment
The Flow Breast Pump Accessory Kit is Class II Medical device and therefore needs to go through rigorous testing to meet the U.S Food and Drug Administration requirements.
Designing protocols and testing equipment to simulate repeated use of the product was essential to passing a federal audit.
Below I briefly cover two pieces of equipment that I designed for validation and verification which was essential to our 510(k) submission to the FDA.
Development Journey
Build Protocols & Sketch fixtures:
The fixtures were designed to rapidly simulate the life cycle of the product.
The first fixture repeatedly boiled batches of the product to simulate rigorous sanitization.
The second fixture carried out thousands pumping sessions while monitoring and recording pressure levels to gauge the duckbill valve service life.
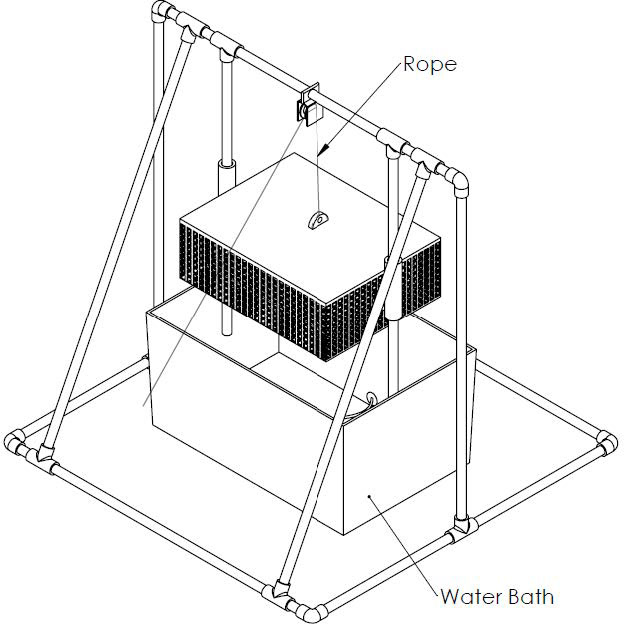
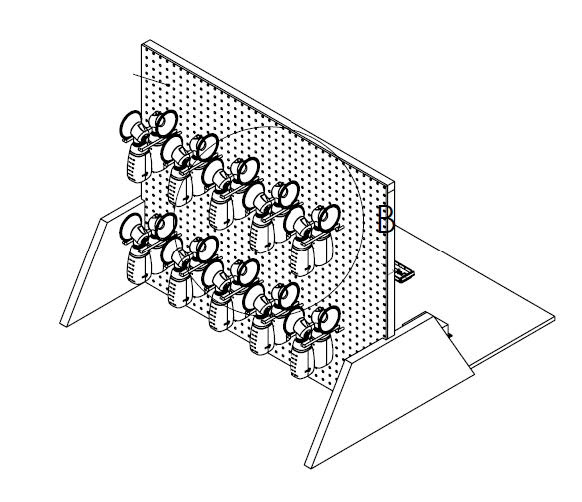
Low Fidelity Prototypes:
We sourced all the electronic components in San Francisco and validated that the code was functional.
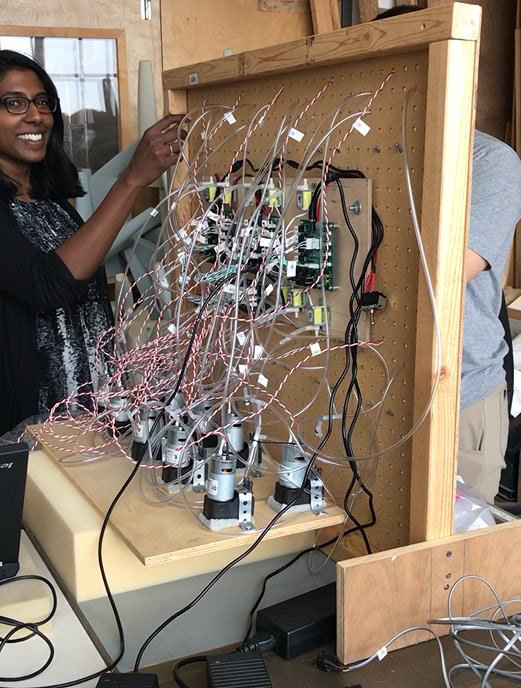

Building Robust Fixtures in China:
I traveled to our supplier and over sought the build of the testing apparatuses that would be used for verifying our product with the FDA.

FDA Clearance:
This fixture along with others helped us clear our 510(k).
